Unlocking the Secrets of How a Car Battery Works
A car battery is a rechargeable device that stores chemical energy and converts it into the electrical energy needed to start your engine and power vehicle systems. It works through a reversible electrochemical reaction between lead plates and sulfuric acid. Understanding its basic operation is key to proper maintenance and avoiding unexpected failures.
You turn the key. You hear that satisfying click-click-VROOM. Your car comes to life. It’s a simple, daily miracle we rarely think about. But what makes it happen? The humble car battery. It’s a heavy box tucked away in the corner of your engine bay, quietly doing its job until one day it doesn’t. That dreaded “click-click-click” of a dead battery is a universal sign of a bad morning.
But have you ever wondered, how does a car battery work? It’s more than just a container of “juice.” It’s a sophisticated chemical reactor, a power reservoir, and the heartbeat of your car’s electrical system. Understanding its secrets doesn’t require an engineering degree. It’s a fascinating story of chemistry, physics, and clever design.
This guide will unlock those secrets. We’ll break down the magic inside that plastic case. You’ll learn what happens during start-up, how it recharges, and why it eventually wears out. By the end, you’ll see your car battery not as a mysterious black box, but as the hard-working component it truly is. Let’s dive in.
In This Article
- 1 Key Takeaways
- 2 📑 Table of Contents
- 3 1. The Heart of the Matter: What is a Car Battery?
- 4 2. The Chemical Magic: How Energy is Made and Stored
- 5 3. The Big Moment: How Your Battery Starts the Car
- 6 4. Teamwork: The Battery and the Charging System
- 7 5. The Slow Fade: Why Car Batteries Eventually Die
- 8 6. Keeping It Alive: Practical Battery Care Tips
- 9 Conclusion: More Than Just a Box
- 10 Frequently Asked Questions
Key Takeaways
- It’s a Chemical Power Plant: A car battery doesn’t “store” electricity; it stores chemical energy that is converted to electrical energy on demand through a reaction.
- The Lead-Acid Reaction is Core: The interaction between lead dioxide (positive plates), sponge lead (negative plates), and sulfuric acid (electrolyte) creates voltage and current.
- Starting is its Biggest Job: The battery must deliver a massive, short burst of power (cold cranking amps) to turn the starter motor and engine.
- It’s Constantly Recharging: The alternator restores the battery’s chemical state after starting and powers the car’s electrical systems while driving.
- Deep Discharge is Damaging: Regularly draining a battery below 50% charge can permanently damage its plates, reducing capacity and lifespan.
- Maintenance Matters: Keeping terminals clean, checking electrolyte levels (on serviceable types), and ensuring secure mounting can prevent many common issues.
- Voltage Tells a Story: A simple multimeter reading (12.6V+ = full, ~12.4V = 75%, 12.0V or less = discharged) is a great diagnostic tool.
📑 Table of Contents
- 1. The Heart of the Matter: What is a Car Battery?
- 2. The Chemical Magic: How Energy is Made and Stored
- 3. The Big Moment: How Your Battery Starts the Car
- 4. Teamwork: The Battery and the Charging System
- 5. The Slow Fade: Why Car Batteries Eventually Die
- 6. Keeping It Alive: Practical Battery Care Tips
- Conclusion: More Than Just a Box
1. The Heart of the Matter: What is a Car Battery?
At its core, a standard car battery is a lead-acid rechargeable battery. Its primary mission is to provide a large, sudden burst of electrical power. This burst is needed for one critical task: cranking the starter motor to turn over the engine. Once the engine is running, a different device (the alternator) takes over to power the car’s systems. The battery then shifts to a backup role, stabilizing the vehicle’s electrical voltage and providing power when demand exceeds the alternator’s output.
Think of it like a water reservoir for a town. The battery is the reservoir, storing a large amount of “water” (energy). Starting the car is like opening a giant floodgate for a few seconds to spin a water wheel. The alternator is the river that constantly refills the reservoir and supplies the town’s daily water needs. The reservoir ensures there’s always enough for an emergency (like starting the car) or a temporary spike in demand.
The Main Components Inside the Box
Pop the lid (on a serviceable battery), and you’ll see the key players:
- Plastic Case: A tough, acid-resistant container that holds everything together.
- Positive and Negative Terminals: The connection points for your car’s cables. They are clearly marked with a “+” and “-“.
- Cells: Inside the case are six separate compartments, called cells. Each cell generates about 2.1 volts. Connected in series (end-to-end), they produce the standard 12.6 volts of a fully charged battery.
- Plates: Inside each cell are stacks of lead plates. They look like grids. There are positive plates coated with lead dioxide (a dark brown paste) and negative plates coated with sponge lead (a gray paste). These plates are the reactive surfaces where chemistry happens.
- Electrolyte: A mixture of sulfuric acid and distilled water that fills the cells and surrounds the plates. This liquid is the crucial medium that allows the chemical reaction to occur.
- Separators: Porous, insulating sheets placed between the positive and negative plates. They prevent the plates from touching and short-circuiting while still allowing the electrolyte to flow freely.
2. The Chemical Magic: How Energy is Made and Stored
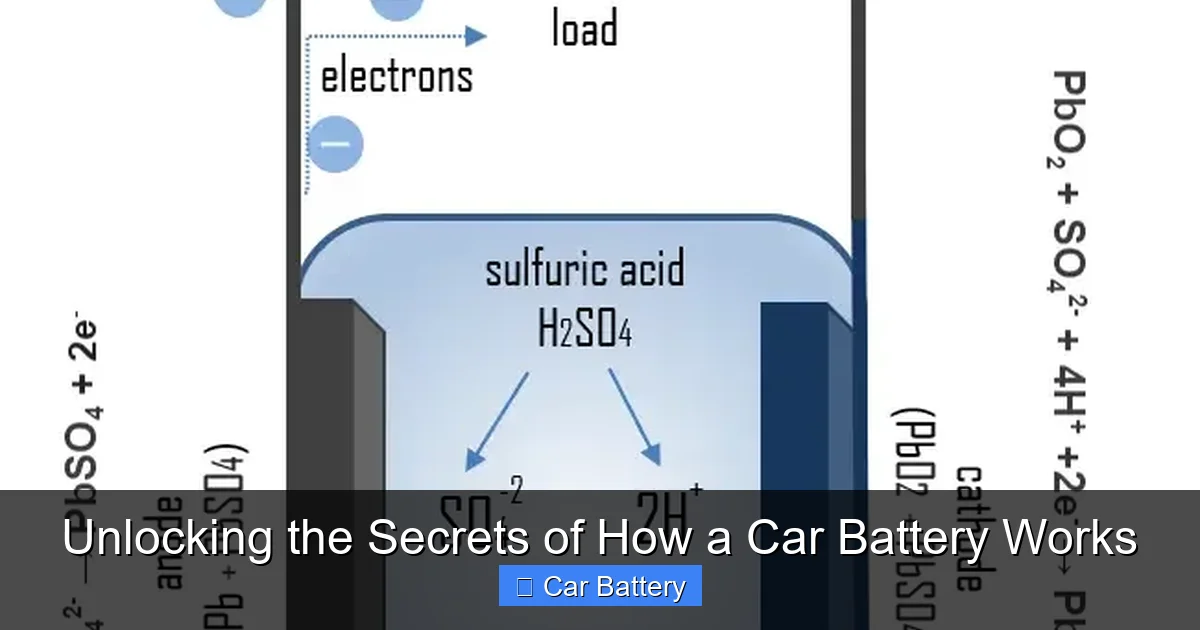
This is where the real secret lies. A battery doesn’t store electricity like a bucket holds water. Instead, it stores chemical potential energy. When you complete a circuit (by turning the key), a chemical reaction is triggered, and this reaction produces electrical energy.
Visual guide about Unlocking the Secrets of How a Car Battery Works
Image source: theengineeringmindset.com
The Discharge Reaction (Using Power)
When you demand power—to start the car or run the radio with the engine off—the battery discharges. Here’s the simplified chemistry:
- The sulfuric acid (H₂SO₄) in the electrolyte reacts with the lead dioxide (PbO₂) on the positive plate and the sponge lead (Pb) on the negative plate.
- This reaction creates lead sulfate (PbSO₄) on both sets of plates and releases water (H₂O) into the electrolyte.
- Critically, this reaction also releases electrons. Electrons flow from the negative terminal, through your car’s circuit (doing work like spinning the starter motor), and back to the positive terminal. This flow of electrons is electric current.
As the battery discharges, the plates become more coated with lead sulfate, and the electrolyte becomes more diluted with water (less acidic). Its ability to produce voltage drops.
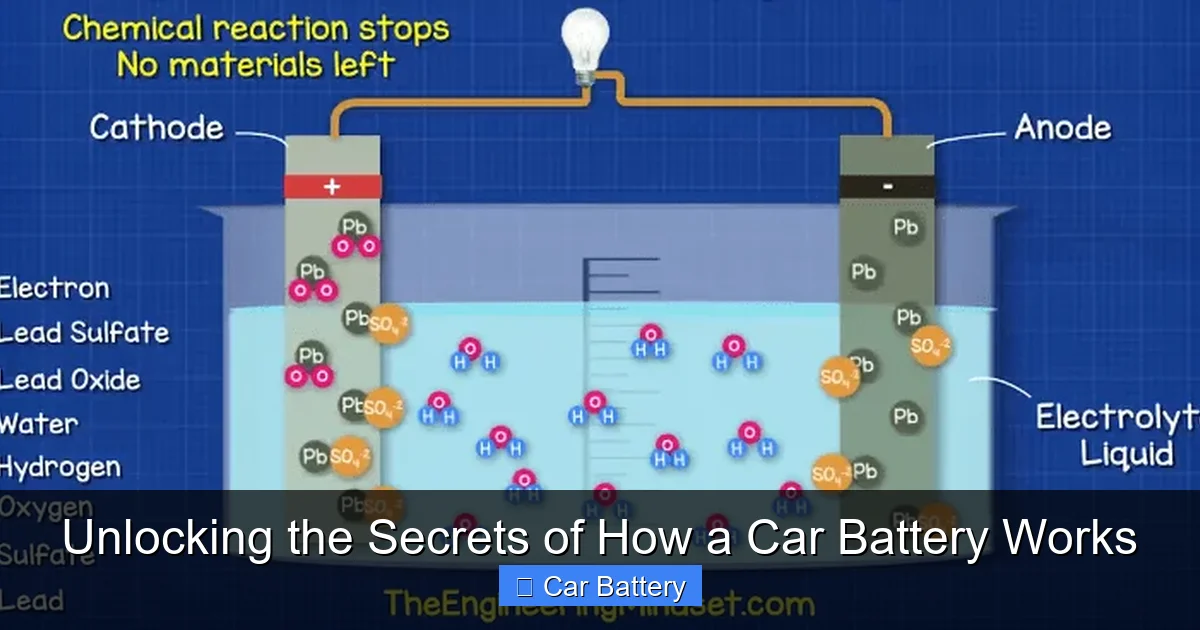
The Charge Reaction (Restoring Power)
This process is beautifully reversible. When the alternator sends current into the battery, it forces the chemical reaction to run in reverse.
- Electrical energy from the alternator is converted back into chemical energy.
- The lead sulfate on the plates breaks down. The sulfate returns to the electrolyte, making it strong acid again.
- The lead dioxide and sponge lead are restored on their respective plates.
- The battery is now ready to discharge again.
This charge/discharge cycle can happen hundreds of times, but not forever. Over time, some of the lead sulfate hardens and won’t convert back, reducing the battery’s capacity. This is a main reason batteries eventually die.
3. The Big Moment: How Your Battery Starts the Car
Starting a car is the single most demanding task for your battery. An engine has immense compression that the starter motor must overcome. This requires a huge amount of torque, which translates to a massive amount of electrical current—often 150 to 300 amps or more for a few seconds.

Visual guide about Unlocking the Secrets of How a Car Battery Works
Image source: theengineeringmindset.com
Cold Cranking Amps (CCA): The Muscle Metric
This is why you see the CCA rating on batteries. CCA measures the number of amps a 12-volt battery can deliver at 0°F (-18°C) for 30 seconds while maintaining at least 7.2 volts. A higher CCA rating means more starting power in cold weather, when engine oil is thick and batteries are less efficient. Choosing a battery with the correct CCA for your climate and vehicle is crucial.
The Starting Sequence
- Key Turn: You turn the key to the “start” position.
- Circuit Complete: This engages the starter solenoid, creating a complete, low-resistance circuit from the battery’s negative terminal, through the car’s chassis (ground), into the starter motor, and back to the battery’s positive terminal.
- Chemical Surge: The sudden demand triggers the discharge reaction in all six cells simultaneously, releasing a flood of electrons (current).
- Motor Spins: This current powers the starter motor, which engages with the engine’s flywheel and cranks the engine.
- Engine Fires: Once the engine starts running on its own combustion, you release the key.
- Job Done: The starter disengages, and the massive current draw stops. The battery, now slightly depleted, awaits recharge from the alternator.
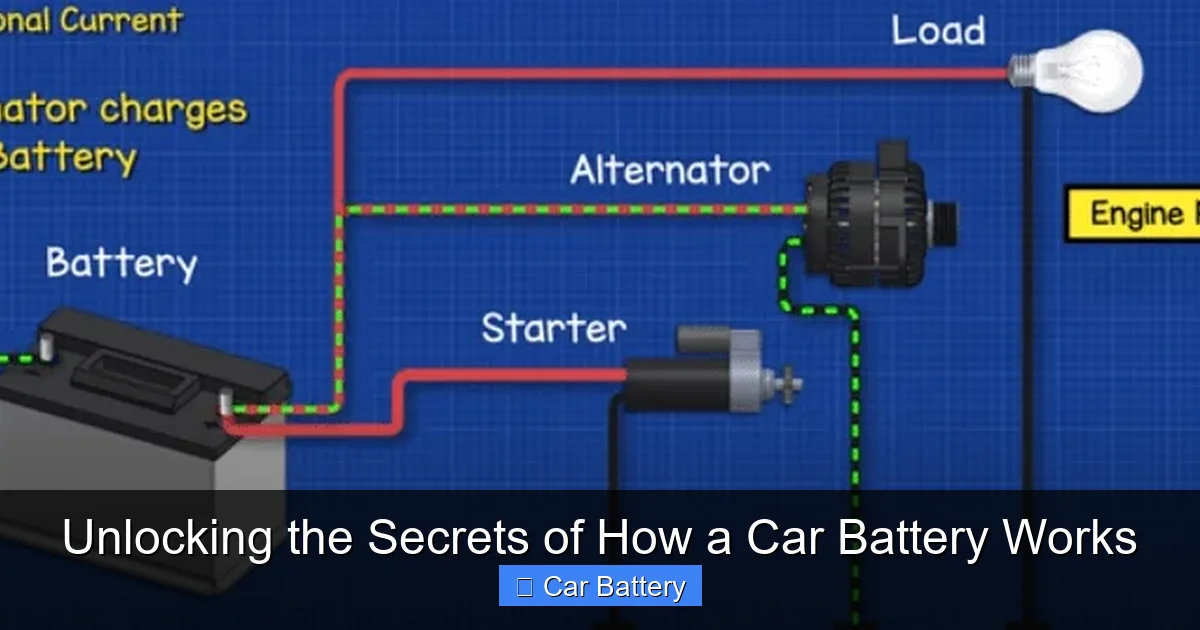
4. Teamwork: The Battery and the Charging System
The battery is not a solo act. It’s the star player in a team called the charging system, which includes the alternator and voltage regulator.
Visual guide about Unlocking the Secrets of How a Car Battery Works
Image source: theengineeringmindset.com
The Alternator’s Role
As soon as the engine runs, the alternator takes over. Driven by a belt from the engine, it converts mechanical energy into electrical energy. This AC current is rectified to DC and used for two things:
- Power All Electrical Accessories: Lights, radio, ECU, fuel pump, windshield wipers—everything runs off the alternator while driving.
- Recharge the Battery: It sends current back into the battery, reversing the discharge reaction and restoring its chemical state.
A Symbiotic Relationship
This relationship is constant. At idle with many accessories on (headlights, A/C, rear defroster), the electrical demand might exceed the alternator’s output. The battery seamlessly supplements power. When demand is low, the alternator focuses on recharging the battery. A faulty alternator will leave the battery to power the entire car, leading to a rapid and complete discharge.
5. The Slow Fade: Why Car Batteries Eventually Die
Even with perfect care, a car battery has a finite life, typically 3-5 years. Here’s what slowly happens inside:
Sulfation: The Primary Killer
Remember the lead sulfate created during discharge? During normal charging, it converts back. But if a battery is left in a partially or fully discharged state (e.g., from a parasitic drain or infrequent use), the soft lead sulfate crystals harden into a stable, crystalline form. This permanent sulfation coats the plates, reducing their active surface area. The battery loses capacity and can no longer accept a full charge.
Plate Corrosion and Grid Growth
The positive plates slowly corrode over many charge cycles. This is a normal wear process. The grids can also physically expand and distort, potentially causing short circuits within a cell.
Electrolyte Loss and Stratification
In non-sealed batteries, water in the electrolyte can evaporate or be broken down by overcharging, exposing the plates to air and causing damage. In all batteries, the electrolyte can “stratify”—the stronger acid sinks to the bottom, leading to uneven wear on the plates.
Physical Damage and Vibration
Loose mounting can let the battery vibrate excessively, which can shake material off the plates or even crack the internal connections and case.
6. Keeping It Alive: Practical Battery Care Tips
Knowing how a car battery works empowers you to take better care of it. Here are practical tips to extend its life:
Regular Visual and Voltage Checks
- Terminals: Keep them clean and tight. Corrosion (a white, blue, or green crust) is an insulator. Clean with a baking soda/water mix and a wire brush.
- Case: Look for cracks, bulges, or leaks.
- Voltage Test: Use a cheap multimeter. With the car off for a few hours: 12.6V+ = fully charged; ~12.4V = 75%; 12.0V = 50% (recharge needed); Below 11.9V = deeply discharged.
Preventing Deep Discharge
- Avoid “Parasitic Drain”: Ensure interior lights, trunk lights, or aftermarket accessories aren’t staying on and draining the battery.
- Drive Regularly: Short trips (under 15-20 minutes) don’t give the alternator enough time to fully recharge the battery after starting.
- Use a Maintainer: If you store a vehicle or drive infrequently, a battery tender or smart maintainer is the best investment you can make. It keeps the battery at 100% without overcharging.
For Serviceable Batteries
If your battery has removable caps, check the electrolyte level every few months (with the engine off!). Top up only with distilled water to just above the plates. Never add acid.
Conclusion: More Than Just a Box
So, the next time you start your car, you’ll know the secret dance happening under the hood. That simple turn of the key sets off a controlled chemical explosion, releasing a torrent of electrons to spin a motor, which starts a series of controlled fuel explosions that power your journey. The car battery is the essential first domino in that chain.
Understanding how a car battery works demystifies a critical part of your vehicle. It helps you diagnose problems, make smarter purchasing decisions, and take simple steps to avoid being stranded. It’s a masterpiece of reliable, reversible electrochemistry that we all depend on. Treat it well, and it will reward you with years of faithful service, always ready for that satisfying VROOM.
Frequently Asked Questions
How long does a car battery typically last?
Most car batteries last between 3 to 5 years. This lifespan depends heavily on climate (extreme heat accelerates wear), driving habits (frequent short trips are hard on it), and maintenance. Regular testing after the 3-year mark is a good idea.
Can a car battery recharge itself?
No, a car battery cannot recharge itself. It requires an external source of DC current to reverse its chemical reaction. While driving, this is provided by the alternator. When parked, a battery charger or maintainer is needed.
What does it mean when my battery is “maintenance-free”?
A maintenance-free (or sealed) battery has a design that minimizes water loss, so it doesn’t require you to add distilled water. It’s fully sealed, except for small vent holes for gas escape. You still need to keep the terminals clean and check the voltage.
Why does a battery die more often in cold weather?
Cold weather thickens engine oil, making the engine harder to crank (requiring more power). At the same time, the chemical reactions inside the battery slow down dramatically, reducing its ability to deliver that power. A weak battery often fails first on a cold morning.
Can I jump-start a completely dead battery?
Yes, you can usually jump-start a dead battery, but success depends on how deeply discharged and how old it is. If it’s simply run down from lights being left on, jumping will work. If it’s old and sulfated, it may not hold a charge even after a jump.
Is a higher CCA battery always better?
Not necessarily. You should match or slightly exceed your vehicle manufacturer’s recommended CCA. A much higher CCA battery is often physically larger and may not fit. In very cold climates, a higher CCA is beneficial, but in moderate climates, the extra capacity isn’t needed and adds cost.






