What Kind of Acid Is in a Car Battery
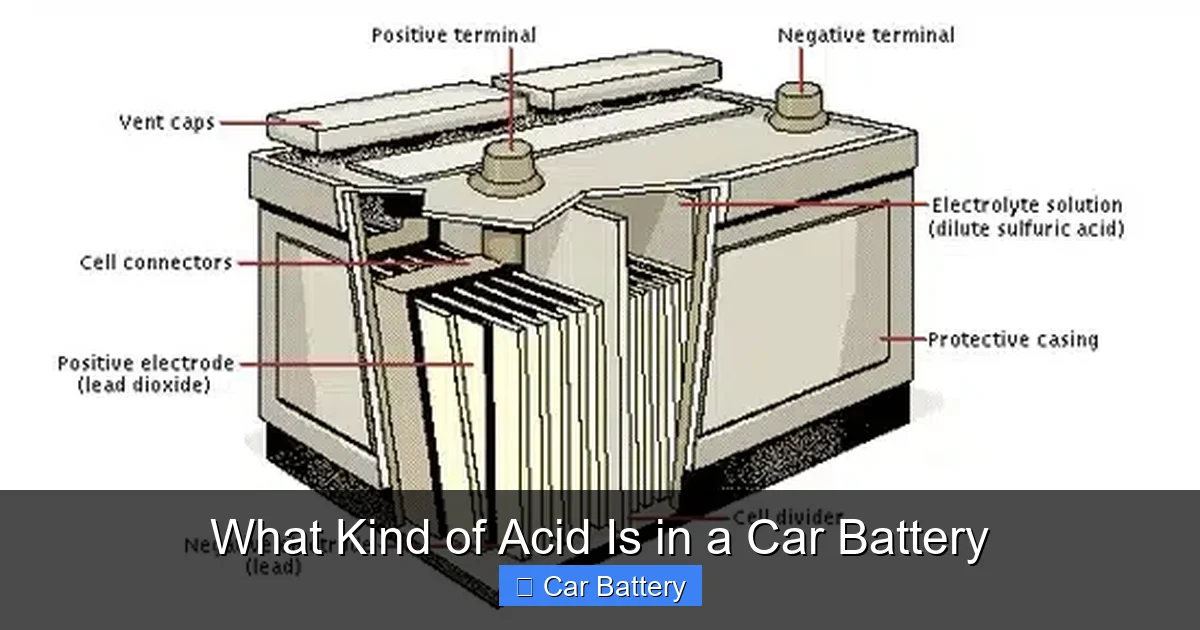
Car batteries contain sulfuric acid, a highly corrosive substance essential for generating electrical power. This acid enables the chemical reaction that produces electricity to start your engine and power vehicle systems.
In This Article
- 1 Key Takeaways
- 2 📑 Table of Contents
- 3 What Kind of Acid Is in a Car Battery?
- 4 Understanding the Chemistry Behind Car Batteries
- 5 Why Sulfuric Acid? The Science of Choice
- 6 Safety Concerns: Handling Sulfuric Acid in Car Batteries
- 7 Maintenance Tips to Protect the Acid and Extend Battery Life
- 8 Modern Batteries: Are They Still Acid-Based?
- 9 Environmental Impact and Recycling
- 10 Conclusion
- 11 Frequently Asked Questions
Key Takeaways
- Sulfuric acid is the primary acid in car batteries: It makes up about 30–50% of the electrolyte solution, mixed with distilled water.
- It enables electrochemical reactions: The acid reacts with lead plates to produce electricity through a process called electrolysis.
- Highly corrosive and dangerous: Direct contact can cause severe burns, so proper handling and protective gear are essential.
- Concentration affects performance: The strength of the acid changes as the battery charges and discharges, impacting voltage and efficiency.
- Safe disposal is critical: Never pour old battery acid down drains; recycle batteries at certified centers to prevent environmental harm.
- Maintenance extends battery life: Checking fluid levels and keeping terminals clean helps maintain optimal acid function and battery health.
- Modern batteries may be sealed: Many newer vehicles use maintenance-free batteries, but the acid is still present inside.
📑 Table of Contents
- What Kind of Acid Is in a Car Battery?
- Understanding the Chemistry Behind Car Batteries
- Why Sulfuric Acid? The Science of Choice
- Safety Concerns: Handling Sulfuric Acid in Car Batteries
- Maintenance Tips to Protect the Acid and Extend Battery Life
- Modern Batteries: Are They Still Acid-Based?
- Environmental Impact and Recycling
- Conclusion
What Kind of Acid Is in a Car Battery?
If you’ve ever popped the hood of your car and seen those two heavy, rectangular boxes with cables attached, you’ve seen a car battery. But what’s really going on inside that sealed (or sometimes vented) unit? The answer lies in a powerful chemical: sulfuric acid. Yes, the same acid used in industrial processes and chemistry labs is quietly working every time you turn the key in your ignition.
Car batteries rely on a chemical reaction to produce electricity, and sulfuric acid is the star player in that reaction. Without it, your car wouldn’t start, your lights wouldn’t turn on, and your radio would stay silent. But this isn’t just any acid—it’s a highly concentrated, corrosive substance that demands respect. Understanding what kind of acid is in a car battery helps you appreciate how your vehicle works and why safety matters when handling or maintaining your battery.
This article will walk you through everything you need to know about the acid in car batteries—what it is, how it works, why it’s dangerous, and how to stay safe. Whether you’re a car enthusiast, a DIY mechanic, or just someone who wants to understand what’s under the hood, this guide will give you the knowledge you need.
Understanding the Chemistry Behind Car Batteries

Visual guide about What Kind of Acid Is in a Car Battery
Image source: carfromjapan.com
To truly grasp what kind of acid is in a car battery, you need to understand how a lead-acid battery works. These batteries—used in most gasoline and diesel vehicles—are based on a simple but brilliant electrochemical design developed in the 19th century. At their core, they use two main materials: lead and lead dioxide, immersed in an electrolyte solution.
The electrolyte is where sulfuric acid comes into play. It’s mixed with distilled water to create a solution that conducts electricity. When the battery is charged, the acid concentration is high. As the battery discharges—like when you start your car—the acid reacts with the lead plates, producing lead sulfate and water. This reaction releases electrons, which flow through the cables to power your vehicle.
How the Chemical Reaction Works
Let’s break it down step by step. Inside the battery, there are multiple cells—usually six in a 12-volt battery—each containing positive and negative lead plates. These plates are submerged in the sulfuric acid solution. When you turn the key, a chemical reaction begins:
– The sulfuric acid (H₂SO₄) splits into hydrogen ions (H⁺) and sulfate ions (SO₄²⁻).
– The sulfate ions react with the lead (Pb) on the negative plate to form lead sulfate (PbSO₄) and release electrons.
– On the positive plate, lead dioxide (PbO₂) reacts with sulfate ions, hydrogen ions, and electrons to form lead sulfate and water.
This flow of electrons from the negative to the positive terminal creates an electric current. That’s the power that starts your engine and runs your electronics.
Charging Reverses the Reaction
When you drive, the alternator recharges the battery by sending current back through it. This reverses the chemical reaction:
– Lead sulfate on both plates breaks down.
– Sulfate ions return to the acid solution.
– Water breaks down into hydrogen and oxygen (which is why some batteries vent gases).
The result? The acid concentration increases again, restoring the battery’s charge. This cycle can repeat hundreds of times, but over time, the plates degrade, and the battery loses efficiency.
Why Sulfuric Acid? The Science of Choice

Visual guide about What Kind of Acid Is in a Car Battery
Image source: carfromjapan.com
You might wonder: why sulfuric acid? Couldn’t another acid work just as well? The answer lies in its unique chemical properties. Sulfuric acid is ideal for car batteries because it’s highly conductive, stable, and reacts efficiently with lead.
High Electrical Conductivity
Sulfuric acid is an excellent conductor of electricity. When dissolved in water, it breaks apart into ions that carry electrical charge. This allows the battery to deliver a strong, steady current—exactly what’s needed to crank a cold engine on a winter morning.
Strong Acid, Strong Reaction
As a strong acid, sulfuric acid fully dissociates in water, meaning it releases a high concentration of hydrogen ions. This makes the electrochemical reaction fast and powerful. Weaker acids wouldn’t provide enough energy to start a car reliably.
Stability and Availability
Sulfuric acid is also chemically stable under normal conditions and widely available. It’s produced in large quantities for industrial use, making it cost-effective for battery manufacturers. Plus, it doesn’t break down easily, so it lasts through many charge-discharge cycles.
Compatibility with Lead
The reaction between sulfuric acid and lead is well understood and controllable. Other metals might react too violently or produce unwanted byproducts. Lead, combined with lead dioxide, creates a predictable and efficient system that’s lasted over 150 years.
Safety Concerns: Handling Sulfuric Acid in Car Batteries
Visual guide about What Kind of Acid Is in a Car Battery
Image source: static.vecteezy.com
Now that you know what kind of acid is in a car battery, it’s crucial to understand the risks. Sulfuric acid is not something to take lightly. It’s highly corrosive and can cause serious injury if mishandled.
Health Hazards of Sulfuric Acid
Direct contact with sulfuric acid can cause severe chemical burns to the skin and eyes. Even small splashes can lead to permanent damage. Inhaling acid fumes—especially in poorly ventilated areas—can irritate the respiratory system and cause coughing, shortness of breath, or lung damage.
If ingested—though unlikely—it can burn the mouth, throat, and stomach, leading to life-threatening complications. That’s why it’s essential to treat every car battery as a potential hazard.
Protective Gear and Safe Practices
If you’re working on a car battery, always wear protective gear:
– Safety goggles to protect your eyes
– Rubber or nitrile gloves to shield your hands
– Long sleeves and pants to cover skin
– Work in a well-ventilated area to avoid inhaling fumes
Never smoke or use open flames near a battery. Charging batteries can release hydrogen gas, which is highly flammable and can explode if ignited.
What to Do in Case of Exposure
If sulfuric acid comes into contact with your skin, immediately flush the area with large amounts of water for at least 15 minutes. Remove contaminated clothing and seek medical attention right away.
If it gets in your eyes, rinse continuously with clean water and get emergency medical help. Do not rub your eyes.
If you inhale fumes, move to fresh air immediately. If breathing is difficult, seek medical care.
Safe Storage and Disposal
Never store old batteries in warm or sunny areas—heat can increase pressure and cause leaks. Always store them upright and out of reach of children and pets.
When it’s time to replace your battery, never throw it in the trash. Car batteries are classified as hazardous waste due to their lead and acid content. Take them to a certified recycling center, auto parts store, or battery retailer. Many places will accept old batteries for free and ensure they’re recycled safely.
Maintenance Tips to Protect the Acid and Extend Battery Life
Even though the acid is sealed inside, proper maintenance can help preserve the electrolyte solution and keep your battery working efficiently for years.
Check Electrolyte Levels (If Applicable)
Some older or serviceable batteries have removable caps that let you check the fluid level. If the plates are exposed, the battery is low and needs distilled water—not tap water, which contains minerals that can damage the cells.
Use a flashlight to look inside. The fluid should cover the plates by about half an inch. If it’s low, add distilled water until it reaches the proper level. Never overfill.
Keep Terminals Clean
Corrosion on battery terminals—those white or greenish crusts—can interfere with electrical connections. This buildup is often a mix of lead sulfate and other chemicals, sometimes accelerated by acid vapors.
To clean:
– Disconnect the negative cable first, then the positive.
– Mix baking soda and water to make a paste.
– Apply with an old toothbrush and scrub the terminals and cable ends.
– Rinse with water and dry thoroughly.
– Reconnect the positive cable first, then the negative.
Apply a thin layer of petroleum jelly or terminal protector spray to prevent future corrosion.
Test Battery Health Regularly
Use a multimeter or visit an auto shop to test your battery’s voltage. A fully charged battery should read around 12.6 volts. If it’s below 12.4 volts, it may need charging or replacement.
Many auto parts stores offer free battery testing. They can also check the specific gravity of the electrolyte using a hydrometer—a tool that measures acid concentration. A low specific gravity reading means the acid is weak and the battery may be failing.
Avoid Deep Discharges
Letting your battery drain completely—like leaving lights on overnight—can damage the plates and reduce the acid’s effectiveness. Try to recharge the battery as soon as possible after a deep discharge.
Modern Batteries: Are They Still Acid-Based?
With the rise of electric vehicles and advanced battery technology, you might wonder if traditional lead-acid batteries are becoming obsolete. The truth is, even in modern cars, lead-acid batteries are still widely used—especially for starting, lighting, and ignition (SLI) functions.
Maintenance-Free and Sealed Batteries
Many newer vehicles use maintenance-free batteries, which are sealed and don’t require fluid checks. These still contain sulfuric acid, but the design minimizes evaporation and spillage. Some use absorbed glass mat (AGM) technology, where the acid is held in fiberglass mats between plates. This makes them more resistant to vibration and allows for deeper discharges.
Hybrid and Electric Vehicles
In hybrid and electric cars, a high-voltage lithium-ion battery powers the motor. However, most still include a 12-volt lead-acid battery to run accessories like lights, computers, and door locks. So even in cutting-edge vehicles, sulfuric acid still plays a role.
The Future of Car Batteries
While lithium-ion and solid-state batteries are gaining ground, lead-acid batteries remain popular due to their low cost, reliability, and recyclability. In fact, over 99% of lead-acid batteries in the U.S. are recycled—the highest rate of any consumer product.
Research continues into safer, more efficient alternatives, but for now, sulfuric acid remains the backbone of most car batteries.
Environmental Impact and Recycling
Because car batteries contain both lead and sulfuric acid, they pose significant environmental risks if not handled properly. Lead is a toxic heavy metal that can contaminate soil and water. Sulfuric acid can lower the pH of waterways, harming aquatic life.
The Importance of Recycling
Recycling car batteries is one of the most successful environmental programs in the world. The lead is recovered and reused to make new batteries. The plastic casing is melted and turned into new cases. The acid is neutralized and treated, or converted into sodium sulfate—a compound used in detergents and fertilizers.
By recycling, we reduce the need for mining raw materials and prevent hazardous waste from entering landfills.
How to Recycle Responsibly
When replacing your battery, ask the retailer if they accept old ones. Most do. You can also search for local recycling centers using tools from the EPA or Earth911. Never dump batteries in the trash or pour acid down the drain—it’s illegal and dangerous.
Conclusion
So, what kind of acid is in a car battery? The answer is sulfuric acid—a powerful, essential component that enables your vehicle to start and run. It’s the heart of the lead-acid battery, driving the chemical reactions that produce electricity. While it’s highly effective, it’s also dangerous and requires careful handling.
Understanding the role of sulfuric acid helps you appreciate the complexity of your car’s electrical system and the importance of proper maintenance. From checking fluid levels to cleaning terminals and recycling old batteries, small actions can make a big difference in performance and safety.
As technology evolves, the future may bring new battery chemistries, but for now, sulfuric acid remains a cornerstone of automotive power. Treat it with respect, maintain your battery wisely, and you’ll keep your car running smoothly for years to come.
Frequently Asked Questions
What type of acid is used in car batteries?
Car batteries use sulfuric acid, which is mixed with distilled water to form the electrolyte solution. This acid is essential for the electrochemical reactions that generate electricity.
Is the acid in car batteries dangerous?
Yes, sulfuric acid is highly corrosive and can cause severe burns to skin and eyes. It also releases flammable hydrogen gas when charging, so proper safety precautions are necessary.
Can I add water to my car battery?
Only if it’s a serviceable battery with removable caps. Add distilled water if the fluid level is low—never tap water, which contains minerals that can damage the battery.
How often should I check my battery’s acid level?
For serviceable batteries, check every 6 months or during routine maintenance. Most modern batteries are sealed and don’t require fluid checks.
What happens if battery acid leaks?
A leak can damage surrounding components and pose a safety hazard. Clean the area with baking soda and water, and replace the battery if it’s compromised.
Can I recycle my old car battery?
Yes, and you should. Take it to an auto parts store, recycling center, or battery retailer. Over 99% of lead-acid batteries are recycled in the U.S.






